모두가 양돈 현장에서 PRRS를 여러 차례 겪어 봤지만, 아직도 PRRS 컨트롤에 대한 '정답'은 없습니다. 오랜 기간 양돈농가를 괴롭혀온 만큼 PRRS에 대한 오해와 편견이 많이 쌓여있는 현실입니다. 'PRRS의 모든 지식'(총 15화)을 통해 우리 농장에 맞는 PRRS 컨트롤의 '해답'을 발견할 수 있길 기대합니다. 본 기고글은 HIPRA 본사에서 출간한 'The book for PRRS Knowledge"' 내용을 번역·정리한 것입니다.
면역 반응에 대한 이해
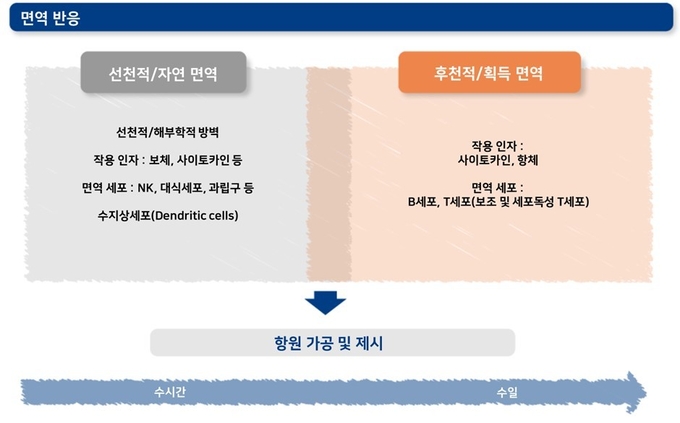
PRRS 바이러스에 대한 면역 반응을 이해하려면 먼저 면역계가 어떻게 작동하는지 살펴봐야 합니다. 학술적인 관점에서는 면역 반응을 선천적 면역과 후천적 면역으로 구분하는 것이 일반적이지만, 이 두 가지 면역 반응은 독립적인 과정이 아닌 밀접한 관계를 가지고 연속적으로 일어나는 과정입니다. 선천적 면역과 후천적 면역의 차이점은 반응 시간, 특이도, 기억 능력으로 요약할 수 있습니다.
선천적 면역 반응은 감염 초기에 즉각적으로 일어납니다. 이 단계에서 세포들은 병원체의 분자학적으로 보존적인 패턴에 반응하여 활성화됩니다. 후천적 면역에는 사이토카인 생성, 세포 독성 작용, 항체 형성 등의 반응들이 해당되며 선천적 면역 반응에 이어 비교적 늦게 활성화됩니다. 이 단계에서는 항원을 가공하고 제시하는 과정(antigen processing and presenting)을 통해 면역 세포들이 항원에 특이적으로 작용할 수 있는 능력을 얻게 됩니다.
활성화된 B세포와 T세포의 일부는 살아남아 후천적 면역의 기억 세포 역할을 담당합니다. 동일한 항원에 다시 노출되는 경우, 남아있던 항원 특이적인 기억 세포들이 빠르게 작동하여 면역계가 이전보다 빠르고 효율적으로 항원에 반응하도록 도와줍니다.
아래 그림은 선천적 면역과 후천적 면역 작용을 요약한 것입니다. 선천적 면역 작용에는 염증 반응과 항원 제시 과정 등 중요한 면역 과정들이 포함됩니다. 항원 가공 및 제시 과정은 정확한 후천성 면역 발달에 필수적인 과정입니다.
선천적 면역 반응
선천적 면역 시스템은 포괄적인 방식으로 병원체를 인지하고 반응하며 면역 반응의 지속 기간이 길지 않습니다. 이러한 특징에서 알 수 있듯 선천적 면역 반응은 감염 및 외부 요인에 대한 빠르고 광범위한 방어력을 제공합니다. 자연 방벽에 의한 방어와 별개로, 선천적 면역 반응에는 체액성 면역(보체, 사이토카인 등)과 세포 매개성 면역 반응이 포함됩니다. 세포 매개성 반응은 호염구(basophil), 호산구(eosinophil), 호중구(neutrophil) 등 과립구와 대식세포(macrophage), 비만세포(mast cell), 수지상세포(dendritic cell), 자연세포독성세포(natural killer cell)의 작용으로 이루어집니다.
선천적 면역 반응을 감염에 대한 일차 저지선으로만 생각할 수 있지만, 이 과정은 후천적 면역이 발달하기 위해 필수적인 과정이기도 합니다. 후천적 면역 반응을 담당하는 T세포들은 스스로 항원을 인지할 수 없기 때문에, 선천적 면역 과정을 통한 항원 제시 과정이 필요합니다. 항원 제시 세포(Antigen presenting cell)에는 수지상세포, 대식세포 및 B세포들이 해당됩니다. 이 중 수지상세포는 가장 강력한 T세포 활성화 작용을 가지며 선천적 면역 반응과 후천적 면역 반응을 연결하는 다리 역할을 하는 것으로 알려져 있습니다.
후천적 면역 반응
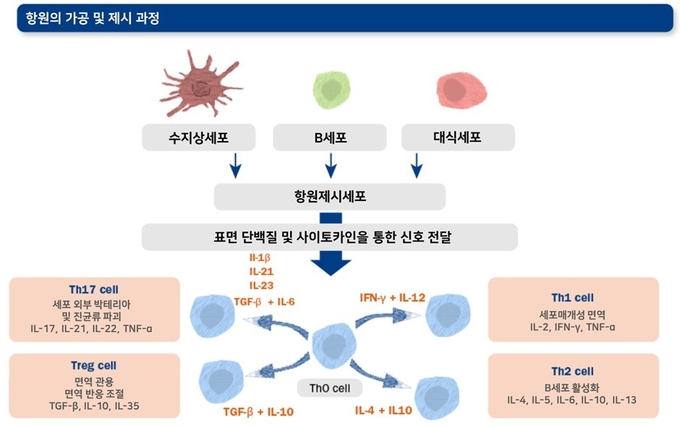
항원 제시 세포들의 표면의 동시 자극 분자들과 분비된 사이토카인들은 신호 역할을 하여 보조 T세포들을 후천적 면역 반응의 핵심적 역할을 수행하는 네 가지 주요 아형(Th1, Th2, Th17, Treg)들로 분화 및 활성화시킵니다.
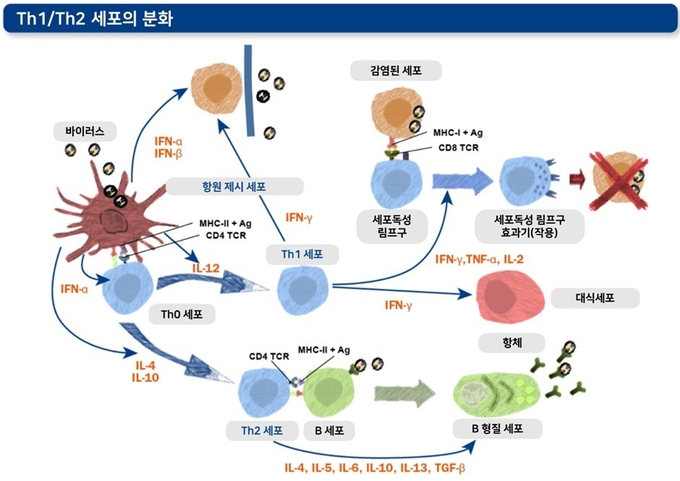
● Th1 세포: IFN-γ를 주로 분비하여 바이러스에 감염된 세포를 죽이는 세포독성 림프구들을 활성화시킴으로써 바이러스 감염을 방어하는 한편, B세포의 항체 형성을 돕는 역할을 수행하기도 합니다. IFN-γ는 대식세포를 활성화시키는 기능도 있습니다.
● Th2 세포: B세포를 ‘항체 공장’ 역할을 하는 B 형질 세포(B plasma cells)로 분화시키는 사이토카인을 분비하여 항체 형성에 핵심적인 작용을 합니다.
● Th17 세포: 호중구와 대식세포를 집결시키고 활성화시켜 세균과 진균 등 외부 병원체들을 방어에 중요한 역할을 수행합니다. Th17 세포는 바이러스 감염 시에도 유도되는 것으로 알려져 있지만, 이들이 바이러스에 대해 어떤 방어 작용을 나타내는지는 아직까지 명확히 밝혀지지 않았습니다.
● Treg 세포 : 이름에서 알 수 있듯 면역 반응을 제어(regulate immune response)하는 세포입니다. 이들은 자가 항원에 대한 면역 반응(자가 면역)이나 병원체에 대한 과도한 면역 반응을 예방합니다.
PRRS 바이러스에 의한 면역 억제
오랜 시간 동안 이어진 과학계의 연구에도 불구하고, PRRS 바이러스의 면역에 대한 핵심적인 내용들은 아직도 명확하게 밝혀지지 않았습니다. 하지만 이 바이러스가 여러 가지 기전을 통해 돼지의 면역 반응을 회피할 수 있다는 것은 잘 알려져 있습니다. PRRS 바이러스는 선천적 면역 반응과 후천적 면역 반응의 다양한 단계들에 강하게 간섭하여 정상적인 면역 반응을 억제합니다.
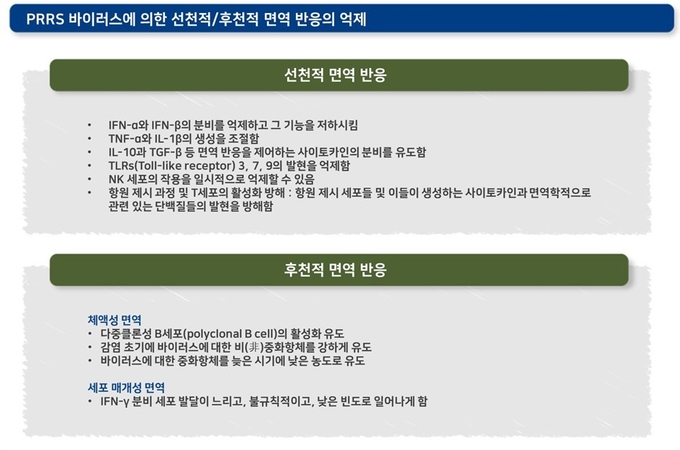
이러한 면역 반응 간섭 작용은 돼지 체내에서 PRRS 바이러스의 증식 기간이 길어지게 하여 바이러스가 다른돼지로 전파될 가능성을 높입니다.
아래 그림에서는 항원 제시 과정과 보조 T세포의 분화 과정에서 PRRS 바이러스에 의해 면역이 억제되는 단계들을 보여주고 있습니다. 여러 단계들에 거쳐 면역 반응을 억제하는 PRRS 바이러스는 돼지의 다른 병원체들에 비해 체액성 면역 및 세포 매개성 면역 반응을 잘 일으키지 않습니다.
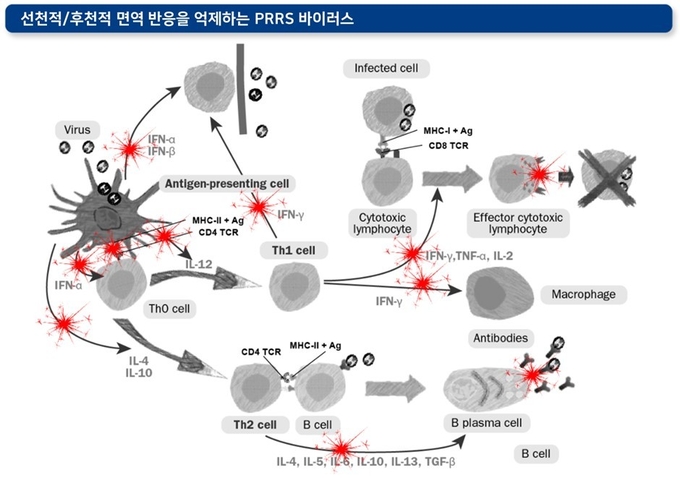
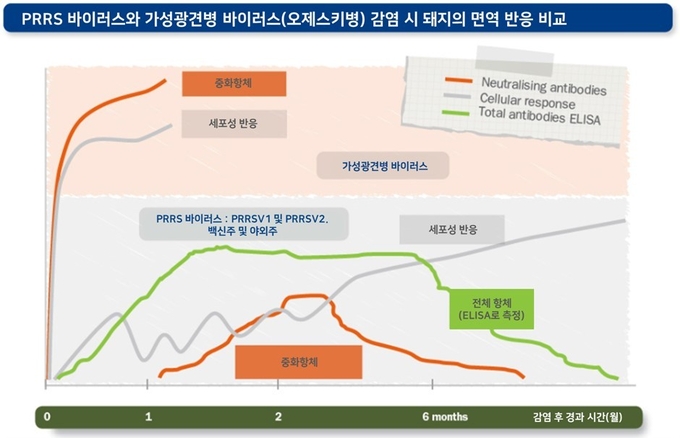
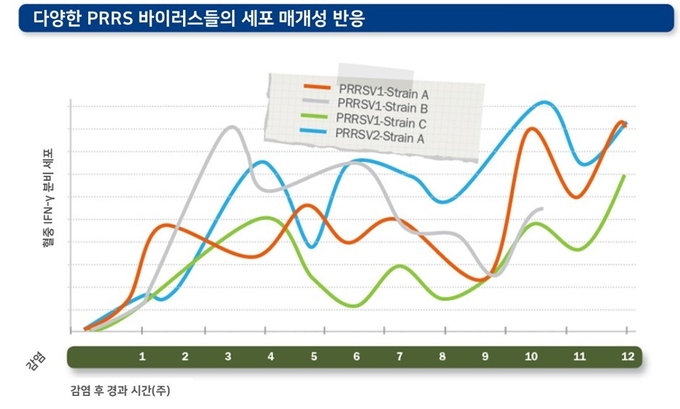
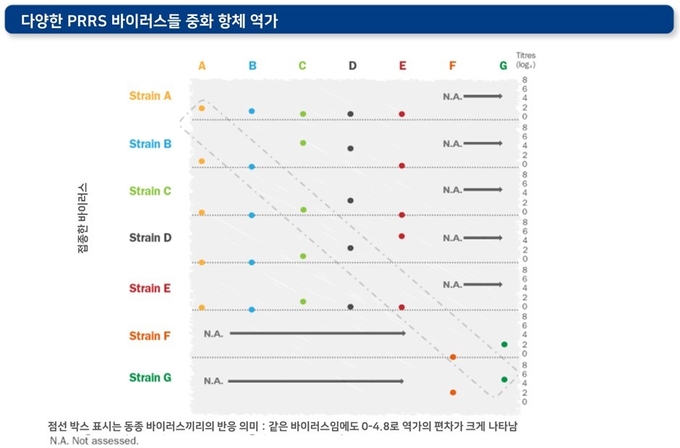
세포 매개성 면역 반응과 중화 항체의 역가는 감염된 PRRS 바이러스에 따라 다양하게 나타나며, 감염된 개체에 따라서도 큰 차이를 보일 수 있습니다. 또한 바이러스 중화항체에 대한 감수성 및 저항성도 각각의 바이러스에 따라 다르게 나타날 수 있습니다.

참고 문헌
-
Albina E, Carrat C, Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res. 1998, 18:485-90.
-
Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006, 80:3994–4004.
-
Badaoui B, Rutigliano T, Anselmo A, Vanhee M, Nauwynck H, Giuffra E, Botti S. RNA-sequence analysis of primary alveolar macrophages after in vitro infection with porcine reproductive and respiratory syndrome virus strains of differing virulence. PLoS One. 2014, 9:e91918.
-
Balasuriya UB, MacLachlan NJ. The immune response to equine arteritis virus: potential lessons for other arteriviruses. Vet Immunol Immunopathol. 2004, 102:107-29.
-
Baumann A, Mateu E, Murtaugh MP, Summerfield A. Impact of genotype 1 and 2 of porcine reproductive and respiratory syndrome viruses on interferon-α responses by plasmacytoid dendritic cells. Vet Res. 2013, 44:33.
-
Bautista EM, Molitor TW. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997, 10: 83-94.
-
Bautista EM, Molitor TW. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch Virol. 1999, 144:1191-200.
-
Buddaert W, Van Reeth K, Pensaert M. In vivo and in vitro interferon (IFN) studies with the porcine reproductive and respiratory syndrome virus (PRRSV). Adv Exp Med Biol. 1998, 440:461-7.
-
Burgara-Estrella A, Díaz I, Rodríguez-Gómez IM, Essler SE, Hernández J, Mateu E. Predicted peptides from non-structural proteins of porcine reproductive and respiratory syndrome virus are able to induce IFN-γ and IL-10. Viruses. 2013, 5:663-77.
-
Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI. Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic. Immunol Res. 2014, 59:81-108.
-
Calzada-Nova G, Schnitzlein WM, Husmann RJ, Zuckermann FA. North American porcine reproductive and respiratory syndrome viruses inhibit type I interferon production by plasmacytoid dendritic cells. J Virol. 2011, 85:2703-13.
-
Calzada-Nova G, Schnitzlein W, Husmann R, Zuckermann FA. Characterization of the cytokine and maturation responses of pure populations of porcine plasmacytoid dendritic cells to porcine viruses and toll-like receptor agonists. Vet Immunol Immunopathol. 2010, 135:20-33.
-
Cancel-Tirado SM, Evans RB, Yoon KJ. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet Immunol Immunopathol. 2004, 102:249-62.
-
Cao J, Grauwet K, Vermeulen B, Devriendt B, Jiang P, Favoreel H, Nauwynck H. Suppression of NK cell-mediated cytotoxicity against PRRSV-infected porcine alveolar macrophages in vitro. Vet Microbiol. 2013, 164:261-9.
-
Chen Z, Zhou X, Lunney JK, Lawson S, Sun Z, Brown E, Christopher-Hennings J, Knudsen D, Nelson E, Fang Y. Immunodominant epitopes in nsp2 of porcine reproductive and respiratory syndrome virus are dispensable for replication, but play an important role in modulation of the host immune response. J Gen Virol. 2010, 91:1047-57.
-
Choi CS, Christianson WT, Collins JE, Joo HS, Molitor T. Antibody dependent enhancement of SIRS virus replication. Am Assoc Swine Pract Newsl. 1992, 4:30.
-
Chung HK, Chae C. Expression of interleukin-10 and interleukin-12 in piglets experimentally infected with porcine reproductive and respiratory syndrome virus (PRRSV). J Comp Pathol. 2003, 129:205-12.
-
Costers S, Vanhee M, Van Breedam W, Van Doorsselaere J, Geldhof M, Nauwynck HJ. GP4-specific neutralizing antibodies might be a driving force in PRRSV evolution. Virus Res. 2010, 154:104-13.
-
Costers S, Lefebvre DJ, Goddeeris B, Delputte PL, Nauwynck HJ. Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet Res. 2009, 40:46.
-
Darwich L, Díaz I, Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Res. 2010, 154:123-32.
-
Darwich L, Gimeno M, Sibila M, Diaz I, de la Torre E, Dotti S, Kuzemtseva L, Martin M, Pujols J, Mateu E. Genetic and immunobiological diversities of porcine reproductive and respiratory syndrome genotype I strains. Vet Microbiol. 2011, 150:49-62.
-
de Lima M, Pattnaik AK, Flores EF, Osorio FA. Serologic marker candidates identified among B-cell linear epitopes of Nsp2 and structural proteins of a North American strain of porcine reproductive and respiratory syndrome virus. Virology. 2006, 30:410–421.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005, 86:1943-51.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006, 351:249-59.
-
Díaz I, Pujols J, Ganges L, Gimeno M, Darwich L, Domingo M, Mateu E. In silico prediction and ex vivo evaluation of potential T-cell epitopes in glycoproteins 4 and 5 and nucleocapsid protein of genotype-I (European) of porcine reproductive and respiratory syndrome virus. Vaccine. 2009, 27:5603-11.
-
Díaz I, Gimeno M, Darwich L, Navarro N, Kuzemtseva L, López S, Galindo I, Segalés J, Martín M, Pujols J, Mateu E. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Vet Res. 2012, 19:43:30.
-
Dokland T. The structural biology of PRRSV. Virus Res. 2010, 154:86-97.
-
Dwivedi V, Manickam C, Binjawadagi B, Linhares D, Murtaugh MP, Renukaradhya GJ. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol J. 2012, 9:45.
-
EvansAB, Loyd H, Dunkelberger JR, van Tol S, Bolton MJ, Dorman KS, Dekkers JCM, Carpenter S. Antigenic and Biological Characterization of ORF2-6 Variants at Early Times Following PRRSV Infection. 2017, 16;9(5). pii: E113. doi: 10.3390/v9050113.
-
Faaberg KS, Hocker JD, Erdman MM, Harris DL, Nelson EA, Torremorell M, Plagemann PG. Neutralizing antibody responses of pigs infected with natural GP5 N-glycan mutants of porcine reproductive and respiratory syndrome virus. Viral Immunol. 2006, 19:294-304.
-
Fan B, Liu X, Bai J, Zhang T, Zhang Q, Jiang P. Influence of the amino acid residues at 70 in M protein of porcine reproductive and respiratory syndrome virus on viral neutralization susceptibility to the serum antibody. Virol J. 2016, 22:51. doi: 10.1186/s12985-016-0505-7.
-
Fang L, Jiang Y, Xiao S, Niu C, Zhang H, Chen H. Enhanced immunogenicity of the modified GP5 of porcine reproductive and respiratory syndrome virus. Virus Genes. 2006, 32:5–11.
-
Gimeno M, Darwich L, Diaz I, de la Torre E, Pujols J, Martín M, Inumaru S, Cano E, Domingo M, Montoya M, Mateu E. Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates. Vet Res. 2011, 18:42:9.
-
Gómez-Laguna J, Salguero FJ, Pallarés FJ, Carrasco L. Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet J. 2013, 195:148-55.
-
Gómez-Laguna J, Salguero FJ, Barranco I, Pallarés FJ, Rodríguez-Gómez IM, Bernabé A, Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J Comp Pathol. 2010, 142:51-60.
-
GuW, Guo L, Yu H, Niu J, Huang M, Luo X, Li R, Tian Z, Feng L, Wang Y. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. J Gen Virol. 2015, 96:1712-22.
-
Han M, Kim CY, Rowland RR, Fang Y, Kim D, Yoo D. Biogenesis of non-structural protein 1 (nsp1) and nsp1-mediated type I interferon modulation in arteriviruses. Virology. 2014, 458-459:136-50.
-
He Q, Li Y, Zhou L, Ge X, Guo X, Yang H. Both Nsp1β and Nsp11 are responsible for differential TNF-α production induced by porcine reproductive and respiratory syndrome virus strains with different pathogenicity in vitro. Virus Res. 2015, 201:32-40.
-
Huang C, Zhang Q, Guo XK, Yu ZB, Xu AT, Tang J, Feng WH. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-κB essential modulator. J Virol. 2014, 88:10934-45.
-
Ke H, Yoo D. The viral innate immune antagonism and an alternative vaccine design for PRRS virus. Vet Microbiol. 2017, 209:75-89.
-
Kim WI, Lee DS, Johnson W, Roof M, Cha SH, Yoon KJ. Effect of genotypic and biotypic differences among PRRS viruses on the serologic assessment of pigs for virus infection. Vet Microbiol. 2007, 123:1-14.
-
Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N. Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine. 2009, 27:3704-18.
-
Kuzemtseva L, de la Torre E, Martín G, Soldevila F, Ait-Ali T, Mateu E, Darwich L. Regulation of toll-like receptors 3, 7 and 9 in porcine alveolar macrophages by different genotype 1 strains of porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2014, 158:189-98.
-
Lager KM, Mengeling WL, Brockmeier SL. Homologous challenge of porcine reproductive and respiratory syndrome virus immunity in pregnant swine. Vet Microbiol. 1997, 58:113-25.
-
Lamontagne L, Page C, Larochelle R, Longtin D, Magar R. Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol Immunopathol. 2001, 82:165-82.
-
Lamontagne L, Page C, Larochelle R, Magar R. Porcine reproductive and respiratory syndrome virus persistence in blood, spleen, lymph nodes, and tonsils of experimentally infected pigs depends on the level of CD8high T cells. Viral Immunol. 2003, 16:395-406.
-
Lee SM, Schommer SK, Kleiboeker SB. Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet Immunol Immunopathol. 2004, 102:217-31.
-
Li Y, Zhu L, Lawson SR, Fang Y. Targeted mutations in a highly conserved motif of the nsp1β protein impair the interferon antagonizing activity of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2013, 94:1972-83.
-
Loemba HD1, Mounir S, Mardassi H, Archambault D, Dea S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1996, 141:751-61.
-
Lohse L, Nielsen J, Eriksen L. Temporary CD8+ T-cell depletion in pigs does not exacerbate infection with porcine reproductive and respiratory syndrome virus (PRRSV). Viral Immunol. 2004, 17:594-603.
-
Lopez OJ, Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004, 102:155-63.
-
Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007, 120:217-29.
-
Lowe JE, Husmann R, Firkins LD, Zuckermann FA, Goldberg TL. Correlation of cell-mediated immunity against porcine reproductive and respiratory syndrome virus with protection against reproductive failure in sows during outbreaks of porcine reproductive and respiratory syndrome in commercial herds. J Am Vet Med Assoc. 2005, 226:1707-11.
-
Lunney JK, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154:1-6.
-
Lunney JK, Fang Y, Ladinig A, Chen N, Li Y, Rowland B, Renukaradhya GJ. Porcine reproductive and respiratory syndrome virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu Rev Anim Biosci. 2016, 4:129-54
-
Magar R, Larochelle R, Nelson EA, Charreyre C. Differential reactivity of a monoclonal antibody directed to the membrane protein of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1997, 61:69-71.
-
Martelli P, Gozio S, Ferrari L, Rosina S, De Angelis E, Quintavalla C, Bottarelli E, Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009, 27:3788-99.
-
Martínez-Lobo FJ, Díez-Fuertes F, Simarro I, Castro JM, Prieto C. Porcine Reproductive and Respiratory Syndrome Virus isolates differ in their susceptibility to neutralization. Vaccine. 2011, 29:6928-40.
-
Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008, 177:345-51.
-
Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003, 309:18-31.
-
Mengeling WL, Lager KM, Vorwald AC, Koehler KJ. Strain specificity of the immune response of pigs following vaccination with various strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2003, 93:13-24.
-
Meulenberg JJ, van Nieuwstadt AP, van Essen-Zandbergen A, Bos-de Ruijter JN, Langeveld JP, Meloen RH. Localization and fine mapping of antigenic sites on the nucleocapsid protein N of porcine reproductive and respiratory syndrome virus with monoclonal antibodies. Virology. 1998, 252:106-14.
-
Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002, 15:533-47.
-
Murtaugh MP, Genzow M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine. 2011, 29:8192-204.
-
Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154:18-30.
-
Nauwynck HJ, Van Gorp H, Vanhee M, Karniychuk U, Geldhof M, Cao A, Verbeeck M, Van Breedam W. Micro-dissecting the pathogenesis and immune response of PRRSV infection paves the way for more efficient PRRSV vaccines. Transbound Emerg Dis. 2012, 1:50-4.
-
Nelson EA, Christopher-Hennings J, Benfield DA. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J Vet Diagn Invest. 1994, 6:410-5.
-
Oleksiewicz MB, Bøtner A, Toft P, Normann P, Storgaard T. Epitope mapping porcine reproductive and respiratory syndrome virus by phage display: the nsp2 fragment of the replicase polyprotein contains a cluster of B-cell epitopes. J Virol. 2001, 75:3277-90.
-
Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002, 76:4241-50.
-
Rose N, Renson P, Andraud M, Paboeuf F, Le Potier MF, Bourry O. Porcine reproductive and respiratory syndrome virus (PRRSv) modified-live vaccine reduces virus transmission in experimental conditions. Vaccine. 2015, 33:2493-9.
-
Pileri E, Gibert E, Soldevila F, García-Saenz A, Pujols J, Diaz I, Darwich L, Casal J, Martín M, Mateu E. Vaccination with a genotype 1 modified live vaccine against porcine reproductive and respiratory syndrome virus significantly reduces viremia, viral shedding and transmission of the virus in a quasi-natural experimental model. Vet Microbiol. 2015, 175:7-16.
-
Pirzadeh B, Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J Gen Virol. 1997, 78: 1867–73.
-
Prieto C, Alvarez E, Martínez-Lobo FJ, Simarro I, Castro JM. Similarity of European porcine reproductive and respiratory syndrome virus strains to vaccine strain is not necessarily predictive of the degree of protective immunity conferred. Vet J. 2008, 175:356-63.
-
Rascón-Castelo E, Burgara-Estrella A, Mateu E, Hernández J. Immunological features of the non-structural proteins of porcine reproductive and respiratory syndrome virus. Viruses. 2015, 7:873-86.
-
Reiner G. Genetic resistance – an alternative for controlling PRRS? Porcine Health Manag. 2016, 2:27.
-
Ren JQ, Sun WC, Lu HJ1, Wen SB, Jing J, Yan FL, Liu H, Liu CX, Xiao PP, Chen X, Du SW, Du R, Jin NY. Construction and immunogenicity of a DNA vaccine coexpressing GP3 and GP5 of genotype-I porcine reproductive and respiratory syndrome virus. BMC Vet Res. 2014, 10:128.
-
Ren J, Lu H, Wen S, Sun W, Yan F, Chen X, Jing J, Liu H, Liu C, Xue F, Xiao P, Xin S, Jin N. Enhanced immune responses in pigs by DNA vaccine coexpressing GP3 and GP5 of European type porcine reproductive and respiratory syndrome virus. J Virol Methods. 2014, 206:27-37.
-
Roca M, Gimeno M, Bruguera S, Segalés J, Díaz I, Galindo-Cardiel IJ, Martínez E, Darwich L, Fang Y, Maldonado J, March R, Mateu E. Effects of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. 2012, 193:92-6.
-
Rodríguez-Gómez IM, Gómez-Laguna J, Carrasco L. Impact of PRRSV on activation and viability of antigen presenting cells. World J Virol. 201, 2:146-51.
-
Royaee AR, Husmann RJ, Dawson HD, Calzada-Nova G, Schnitzlein WM, Zuckermann FA, Lunney JK. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol. 2004, 102:199-216.
-
Samsom JN, de Bruin TG, Voermans JJ, Meulenberg JJ, Pol JM, Bianchi AT. Changes of leukocyte phenotype and function in the broncho-alveolar lavage fluid of pigs infected with porcine reproductive and respiratory syndrome virus: a role for CD8(+) cells. J Gen Virol. 2000, 81:497-505.
-
Scortti M, Prieto C, Martínez-Lobo FJ, Simarro I, Castro JM. Effects of two commercial European modified-live vaccines against porcine reproductive and respiratory syndrome viruses in pregnant gilts. Vet J. 2006, 172:506-14.
-
Shimizu M, Yamada S, Kawashima K, Ohashi S, Shimizu S, Ogawa T. Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet Immunol Immunopathol. 1996, 50:19-27.
-
Silva-Campa E, Mata-Haro V, Mateu E, Hernández J. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs). Virology. 2012, 430:73-80.
-
Sun Y, Han M, Kim C, Calvert JG, Yoo D. Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses. 2012, 4:424-46.
-
Takikawa N, Kobayashi S, Ide S, Yamane Y, Tanaka Y, Yamagishi H. Detection of antibodies against porcine reproductive and respiratory syndrome (PRRS) virus in swine sera by enzyme-linked immunosorbent assay. J Vet Med Sci. 1996, 56: 355–357.
-
van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. 2003, 21:1952-7.
-
Vanhee M, Van Breedam W, Costers S, Geldhof M, Noppe Y, Nauwynck H. Characterization of antigenic regions in the porcine reproductive and respiratory syndrome virus by the use of peptide-specific serum antibodies. Vaccine. 2011, 29:4794-804.
-
Vanhee M, Costers S, Van Breedam W, Geldhof MF, Van Doorsselaere J, Nauwynck HJ. A variable region in GP4 of European-type porcine reproductive and respiratory syndrome virus induces neutralizing antibodies against homologous but not heterologous virus strains. Viral Immunol. 2010, 23:403-13.
-
Van Reeth K, Labarque G, Nauwynck H, Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res Vet Sci. 1999, 67:47-52.
-
Vézina SA, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1996, 60:94-9.
-
Walsh KP, Mills KH. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013, 34:521-30.
-
Wang G, Song T, Yu Y, Liu Y, Shi W, Wang S, Rong F, Dong J, Liu H, Cai X, Zhou EM. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2011, 142:170-8.
-
Wang R, Nan Y, Yu Y, Yang Z, Zhang YJ. Variable interference with interferon signal transduction by different strains of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 2013, 166:493-503.
-
Weesendorp E, Morgan S, Stockhofe-Zurwieden N, Popma-De Graaf DJ, Graham SP, Rebel JM. Comparative analysis of immune responses following experimental infection of pigs with European porcine reproductive and respiratory syndrome virus strains of differing virulence. Vet Microbiol. 2013, 163:1-12.
-
Yang L, Frey ML, Yoon KJ, Zimmerman JJ, Platt KB. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M, GP5 and GP3 proteins and susceptibility to neutralization. Arch Virol. 2000, 145: 1599–619.
-
Yoon IJ, Joo HS, Christianson WT, Kim HS, Collins JE, Morrison RB, Dial GD. An indirect fluorescent antibody test for the detection of antibody to swine infertility and respiratory syndrome virus in swine sera. J Vet Diagn Inv. 1992, 4:144–7.
-
Yoon IJ, Joo HS, Goyal SM, Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994, 6:289-92.
-
Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill HT, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995, 7:305-12.
-
Yoon KJ, Wu LL, Zimmerman JJ, Hill HT, Platt KB. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996, 9:51-63.
-
Yoon KJ, Wu LL, Zimmerman JJ, Platt KB. Field isolates of porcine reproductive and respiratory syndrome virus (PRRSV) vary in their susceptibility to antibody dependent enhancement (ADE) of infection. Vet Microbiol. 1997, 55:277-87.
-
Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: 10th ed. Diseases of swine, Ed. Wiley-Blackwell. 2012, 31:463-86.