모두가 양돈 현장에서 PRRS를 여러 차례 겪어 봤지만, 아직도 PRRS 컨트롤에 대한 '정답'은 없습니다. 오랜 기간 양돈농가를 괴롭혀온 만큼 PRRS에 대한 오해와 편견이 많이 쌓여있는 현실입니다. 'PRRS의 모든 지식'(총 15화)을 통해 우리 농장에 맞는 PRRS 컨트롤의 '해답'을 발견할 수 있길 기대합니다. 본 기고글은 HIPRA 본사에서 출간한 'The book for PRRS Knowledge"' 내용을 번역·정리한 것입니다.

호흡기복합감염증(PRDC)의 기저 원인 PRRS바이러스
PRRS 바이러스는 돼지에게 다른 병원체의 감염에 대한 감수성을 높이며, PRRS 바이러스와 다른 병원체가 동시에 감염될 수도 있습니다. 양돈 현장에서는 PRRS 발생 시 다른 세균이나 바이러스와의 복합감염이 매우 흔하게 발생합니다. PRRS 바이러스는 보통 다른 병원체와의 복합감염 형태로 증상이 발현되어, 진단시기를 늦추고 문제 해결과정을 복잡하고 어렵게 만듭니다. 이러한 현상은 특히 이유자돈이나 육성돈 구간에서 두드러집니다.
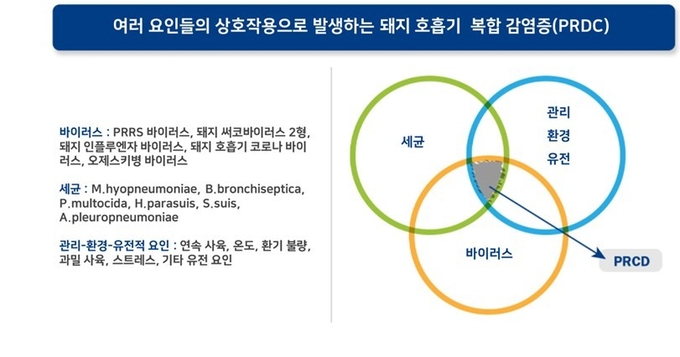
바이러스 및 세균성 병원체의 복잡한 상호작용으로 나타나는 다인성 호흡기질병을 정의하기 위해 ‘돼지호흡기복합감염증(PRDC, Porcine respiratory disease complex)’이라는 용어가 사용되고 있습니다. PRDC의 원인은 여러 병원체의 복합감염뿐 아니라 관리, 사육환경, 유전인자 등의 여러 요인을 포괄적으로 호흡기질병의 원인으로 보고 접근합니다. 이러한 여러 원인이 복합적으로 작용하는 PRDC는 대표적으로 식욕부진, 성장지연, 발열, 기침, 호흡곤란 등의 증상을 통해 의심해볼 수 있습니다.
PRRS 바이러스는 어떻게 PRDC를 유발할까?
PRRS 바이러스가 돼지에 다른 병원체들의 감염 감수성을 높이고 복합감염을 유도하는 원리는 다음과 같습니다.
①PRRS 바이러스는 호흡기도의 미세 구조를 손상시키고, 호흡기의 세균 제거 기능(bacterial clearance)을 방해합니다.
②폐포 대식세포(Alveolar Macrophage)는 폐에서 호흡기 면역에 주요 역할을 하는 세포인 동시에 PRRS 바이러스 일차적으로 감염을 노리는 표적 세포입니다. 특히 PRRS 바이러스는 식균작용을 왕성하게 하며 능력을 최대로 발휘하는 폐포 대식세포에서 증식하면서 이들을 파괴합니다.
③PRRS 바이러스는 폐포 대식세포 외 다른 항원제시 세포(antigen-presenting cell)에도 영향을 미쳐 면역형성의 초기 능력을 저해합니다.
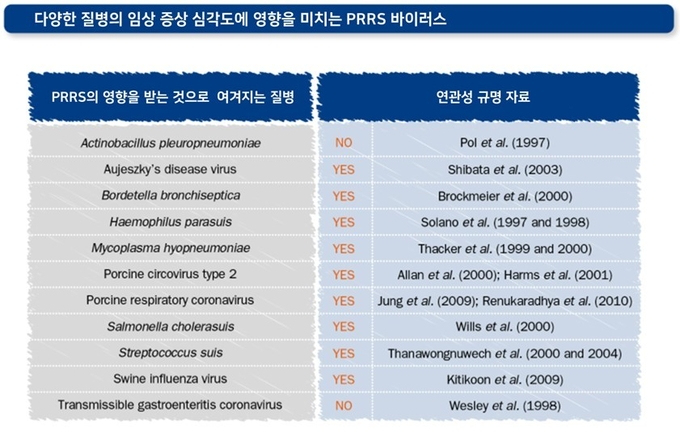
PRRS 바이러스는 아래와 같은 기전으로 면역세포에 다양한 영향을 미쳐 돼지의 면역 반응을 교란하고, 결과적으로 세균 및 바이러스에 대한 대응능력을 낮춰 PRDC를 유발하게 됩니다. 또한 이러한 면역 억제수준은 감염된 PRRS 바이러스주(strain)에 따라 각기 다르게 나타납니다.
●직·간접적인 세포사멸 및 괴사를 유발하여 감염된 대식세포는 물론 감염되지 않은 대식세포까지 사멸시킴
●대식세포의 살균기능을 감소시킴
●I형 인터페론 등 광범위 항바이러스 단백질을 억제하여 다른 바이러스의 감염 가능성을 증가시킴
●전염증성 및 항염증성 사이토카인의 불균형을 유발함
●특정 사이토카인의 분비를 유도하여 면역 반응을 악화시킴
●정확한 항원제시 기능을 방해함
●세포매개성 면역에 관여하는 NK 세포의 세포독성을 감소시킴
PRRS에 의한 경제적 피해는 어느 정도일까?
유럽에서의 PRRS에 의한 피해
다수의 연구결과를 종합해보면 유럽 농장에서 PRRS 발병에 의한 평균 손실액은 모돈 1두당 약 100-200유로(135,000-270,000원) 정도로 평가하고 있습니다. 이는 유럽의 돈가를 고려했을 때 농가에 상당한 부담을 주는 경제적 피해로 볼 수 있습니다.
독일 연구팀이 9개 농장에서의 PRRS 발병에 따른 피해를 분석한 결과, 모돈 1두당 출하 자돈수가 1.7두(-18%) 감소했음을 확인하였습니다. 더불어 평균 18주 동안 지속된 PRRS 발병기간 내 추가적인 치료, 진단, 노동력 발생에 따른 부가적인 손실액은 모돈 두당 59-379유로(평균 126유로, 약 17만원)으로 분석되었습니다. 해당 농장들에서 공통적으로 호소한 피해는 실산자수의 감소(매 분만 복당 2~17%, 평균 8% 감소), 이유 전 폐사율 증가(17~124%, 평균 36% 증가), 이유 후 폐사율 증가(85~350%, 평균 167% 증가)가 있었습니다.
연구팀에 따르면 PRRS에 의한 경제적 피해는 (1)발병한 PRRS 바이러스주(strain)의 병원성, (2)농장의 관리 수준, (3)농장에서 선택한 PRRS 컨트롤 전략의 크게 세가지 요인에 따라 다르게 나타났습니다.
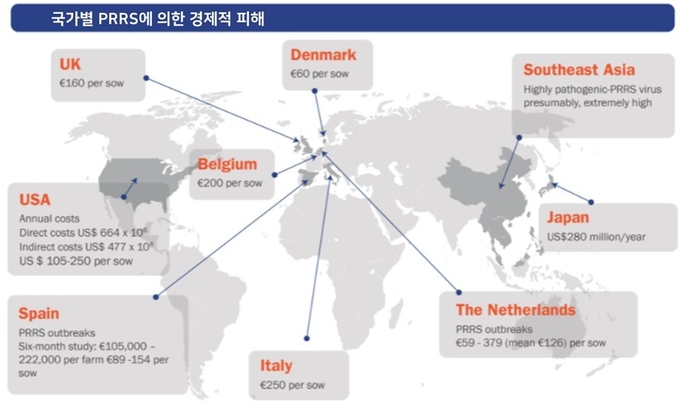
유럽 다른 국가의 연구에서도 비슷한 수준으로 피해가 보고되었습니다. 스페인 농장들의 PRRS 발병 사례들을 분석한 결과, 모돈 2,500두 규모의 일괄사육 농장에서 PRRS 발병 후 6개월 동안 22만 2천 유로(약 3억 원)의 손실액이 발생했습니다. 또한 양성 불안정 농장(신생자돈에서 PRRS 바이러스 혈증이 확인됨)의 경우 뚜렷한 임상증상이 없는 상황에서도 모돈 1두당 연간 30~50유로(4만원-6만8천원)의 경제적 피해가 발생했습니다.
미국에서의 PRRS에 의한 피해
2013년 미국의 연구팀은 PRRS에 의한 미국의 연간 손실액이 664백만 달러(분석기간: 2005~2010년)로 7,636억 원에 달할 것으로 추정했습니다. 이는 2005년에 다른 연구에서 발표한 100백만 달러(약 1150억 원)보다 높은 추정치입니다. 두 연구에서는 항목별 손실액의 비율에서 명확한 차이를 보였는데, 전체 손실액에서 번식 피해가 차지하는 비중이 2005년 연구에서는 12%에 불과했던 반면, 2013년 연구에서는 45%에 달했습니다.
2013년의 연구에 따르면, 백신 접종, 차단방역 강화 등 PRRS 발병에 기인한 간접비용은 연간 477백만 달러(약 5,485억 원)으로 분석되었습니다. 따라서, 미국의 PRRS에 의한 직접적 손실액과 간접적 비용은 연간 10억 달러(약 1조 1천억 원) 이상일 것으로 추정됩니다.
일부 연구자들은 PRRS에 의한 이유두수 감소가 연간 8백만 두에 달한다고 추정하기도 합니다. 캐나다와 멕시코에서의 피해도 미국과 유사할 것으로 여겨집니다.
아시아에서 PRRS에 의한 경제적 피해
일본의 연구팀은 일본 내 PRRS에 의한 연간 손실액을 280백만 달러(3,320억 원)으로 분석했습니다. 이 분석에는 이유 전(+6.7%), 이유 후 자돈(+30.7%), 비육돈(+13.7%)에서의 폐사율 증가와 유산(+36.4%) 및 사산(+6.6%) 비율의 증가와 같은 다양한 항목이 포함되었습니다. 일당 증체량 역시 5.8% 감소하는 것으로 분석되었습니다.
아시아 지역에서 고병원성 PRRS 바이러스(HP-PRRSV)의 등장으로 PRRS에 의한 경제적 피해는 더욱 증가하고 있습니다. 예를 들어, 베트남에서 고병원성 PRRS 유행은 ASF가 발생하기 전까지 다른 어떤 질병들보다 돈육 공급시장에 큰 영향을 미치는 요인이었습니다.
전 세계 양돈산업에 분포한 PRRS 바이러스
PRRS 바이러스는 유전형에 따라 유럽형(type 1)과 북미형(type 2)로 구분됩니다. 유럽형 바이러스는 유럽 지역에서, 북미형 바이러스는 북미 지역에서 최초로 보고되어 이와 같이 명명되었습니다. 현재에는 이들 두 유전형을 PRRSV1과 PRRSV2라는 두 개의 유전형으로 분류하고 있습니다.
PRRSV1의 경우 최소 4개의 아형(subtype)이 보고되었는데, 서유럽 지역에서 주로 발생하는 subtype I, 폴란드 및 그 동쪽에 위치한 일부 국가에서만 발생하는 subtype II, III, IV가 그것입니다. PRRSV2의 경우 2개의 계통(clade)로 분류되고 있습니다. PRRSV1과 PRRSV2는 모두 전 세계적으로 퍼져 있습니다. 유럽형과 북미형으로 분류되던 PRRS 바이러스는 지역과 무관하게 확산되었으며, 호주, 뉴질랜드, 스칸디나비아 국가들, 스위스 및 일부 남미 국가 등 PRRS 청정 국가들 이외에는 돼지를 사육하는 모든 나라에서 발생합니다.
PRRS 바이러스가 변이를 거듭하면서 높은 병원성을 가진 변이주가 발생하기도 합니다. 중국에서 큰 피해를 유발한 고병원성 PRRS 바이러스는 PRRSV2에 속하는 바이러스입니다. 이 바이러스는 아시아 지역에 빠르게 전파되었으며, 현재는 부탄, 캄보디아, 중국, 인도네시아, 라오스, 말레이시아, 미얀마, 필리핀, 러시아, 싱가포르, 베트남 등 아시아 지역의 여러 국가에서 발생이 보고되었습니다(OIE database, 2013). PRRSV1에 속하는 바이러스에서도 높은 병원성과 폐사율을 유발하는 변이주가 발생하고 있습니다. subtype III에 속하는 Lena strain이 대표적인 고병원성 PRRSV1로 알려져 있습니다.
한국을 비롯한 아시아 지역에서는 PRRSV1과 PRRSV2의 혼합감염이 흔하게 발생되고 있습니다. 한국에서는 90년대 중반에 PRRSV2(북미형)이 확인되고 2000년대 초반에 PRRSV1(유럽형)이 보고된 이후 두 유전형의 PRRS 바이러스에 혼합감염이 확인된 농장의 비율이 점차 높아지고 있습니다. 국내 연구팀이 2013년부터 2016년까지 전국 631개 농장의 PRRS 바이러스 분포를 조사한 결과 PRRSV1(유럽형) 단일감염이 38.4%, PRRSV2(북미형) 단일감염이 37.4%로 비슷한 수준으로 확인되었으며, PRRSV1(유럽형)과 PRRSV2(북미형)이 동시에 감염된 농장도 전체의 24.2%로 상당히 높은 비율을 보여줬습니다(Kang et al, 2018).
2007년부터 2008년까지의 국내 PRRS 감염 상황을 조사한 이전의 연구 결과(Lee et al, 2010)에서 PRRSV1(유럽형) 단일감염이 29.4%, PRRSV2(북미형) 단일감염이 54.4%, PRRSV1(유럽형)과 PRRSV2(북미형)의 동시감염이 16.2%였던 것과 비교해보면, PRRSV1(유럽형)과 PRRSV2(북미형)의 혼합감염 사례는 지속적으로 증가하는 추세를 보이고 있습니다.

PRRS 바이러스의 세계적 분포는 지속적으로 바뀌고 있으며, 중국에서 발생한 고병원성 PRRS 바이러스가 아시아 주변국가로 확산된 것과 같이 PRRS 바이러스의 대륙간·국가간 전파 위험은 현재 진행형입니다. 특히 최근 칠레에서 관찰된 사례와 같이, PRRS 청정국에서 바이러스가 유입될 경우 해당국가의 양돈산업에 재앙적인 결과를 초래할 수 있습니다.

참고 문헌
-
Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000, 145:2421-9.
-
Baron T, Albina E, Leforban Y, Madec F, Guilmoto H, Plana Duran J, Vannier P. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann Rech Vet. 1992, 23:161-6.
-
Benfield DA, Nelson E, Collins JE, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 1992. 4, 127-133.
-
Benfield D, Nelson J, Rossow K, Nelson C, Steffen M, Rowland R. Diagnosis of persistent or prolonged porcine reproductive and respiratory syndrome virus infections Vet Res. 2000, 31:71.
-
Blaha T. The ”colorful” epidemiology of PRRS. Vet Res BioMed Central. 2000, 31:77-83.
-
Brockmeier SL, Palmer MV, Bolin SR. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. Am J Vet Res. 2000, 61:892-9.
-
Carman S, Sanford SE, Dea S. Assessment of seropositivity to porcine reproductive and respiratory syndrome (PRRS) virus in swine herds in Ontario–1978 to 1982. Can Vet J. 1995, 36:776-7.
-
Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992, 4:117-26.
-
Cortey M, Díaz I, Martín-Valls GE, Mateu E. Next-generation sequencing as a tool for the study of Porcine reproductive and respiratory syndrome virus (PRRSV) macro- and micro- molecular epidemiology. Vet Microbiol. 2017. doi: 10.1016/j.vetmic.2017.02.002.
-
Delrue I, Van Gorp H, Van Doorsselaere J, Delputte PL, Nauwynck HJ. Susceptible cell lines for the production of porcine reproductive and respiratory syndrome virus by stable transfection of sialoadhesin and CD163. BMC Biotechnol. 2010, 29:10:48.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006, 351:249-59.
-
Dobrescu I, Levast B, Lai K, Delgado-Ortega M, Walker S, Banman S, Townsend H, Simon G, Zhou Y, Gerdts V, Meurens F. In vitro and ex vivo analyses of co-infections with swine influenza and porcine reproductive and respiratory syndrome viruses. Vet Microbiol.2014, 169:18-32.
-
Duan X, Nauwynck HJ, Pensaert MB. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol. 1997, 56:9-19.
-
Fan P, Wei Y, Guo L, Wu H, Huang L, Liu J, Liu C. Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Virol J.2013, 10:265.
-
Forsberg R, Oleksiewicz MB, Petersen AM, Hein J, Bøtner A, Storgaard T. A molecular clock dates the common ancestor of European-type porcine reproductive and respiratory syndrome virus at more than 10 years before the emergence of disease. Virology. 2001, 289:174-9.
-
Gómez-Laguna J, Salguero FJ, Pallarés FJ, Carrasco L. Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet J. 2013, 195:148-55.
-
Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001, 38:528-39.
-
He Q, Li Y, Zhou L, Ge X, Guo X, Yang H. Both Nsp1β and Nsp11 are responsible for differential TNF-α production induced by porcine reproductive and respiratory syndrome virus strains with different pathogenicity in vitro. Virus Res. 2015, 201:32-40.
-
Hill H. Overview and history of Mystery Swine Disease (swine infertility/respiratory syndrome). Proceedings of the Mystery Swine Disease Committee Meeting, Livestock Conversation Institute, Denver, CO. 1990, 29–31.
-
Hirose 0, Kudo H, Yoshizawa S, Hiroike T, Nakane T. Prevalence of porcine reproductive and respiratory syndrome virus in Chiba prefecture. J Jpn Vet Med Assoc. 1995, 48:650-3.
-
Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL and Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013, 21:72-84.
-
Hirose O, Shibata I, Kudou H, Samegai Y, Yoshizawa S, Ono M, Nishimura M, Hiroike T, Kageyama K, Sakano T. Experimental infection of SPF piglets with porcine reproductive and respiratory syndrome (PRRS) viruses isolated from two farms. J Vet Med Sci. 1995, 57:991-5.
-
Jung K, Renukaradhya GJ, Alekseev KP, Fang Y, Tang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009, 90:2713-23.
-
Karniychuk UU, Nauwynck HJ. Quantitative changes of sialoadhesin and CD163 positive macrophages in the implantation sites and organs of porcine embryos/fetuses during gestation. Placenta. 2009, 30:497-500.
-
Karniychuk UU, Geldhof M, Vanhee M, Van Doorsselaere J, Saveleva TA, Nauwynck HJ. Pathogenesis and antigenic characterization of a new East European subtype 3 porcine reproductive and respiratory syndrome virus isolate. BMC Vet Res. 2010, 4:6:30.
-
Karniychuk UU, Nauwynck HJ. Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet Res. 2013, 44:95.
-
Kavanová L, Matiašková K, Levá L, Štěpánová H, Nedbalcová K, Matiašovic J, Faldyna M, Salát J. Concurrent infection with porcine reproductive and respiratory syndrome virus and Haemophilus parasuis in two types of porcine macrophages: apoptosis, production of ROS and formation of multinucleated giant cells. Vet Res.2017, 48:28.
-
Kitikoon P, Vincent AL, Jones KR, Nilubol D, Yu S, Janke BH, Thacker BJ, Thacker EL. Vaccine efficacy and immune response to swine influenza virus challenge in pigs infected with porcine reproductive and respiratory syndrome virus at the time of SIV vaccination. Vet Microbiol. 2009, 139:235-44.
-
Keffaber KK. Reproductive failure of unknown etiology. AASP. 1989, 1: 2–10.
-
Lawson SR, Rossow KD, Collins JE, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus infection of gnotobiotic pigs: sites of virus replication and co-localization with MAC-387 staining at 21 days post-infection. Virus Res. 1997, 51:105-13.
-
Leng X, Li Z, Xia M, Li X, Wang F, Wang W, Zhang X, Wu H. Mutations in the genome of the highly pathogenic porcine reproductive and respiratory syndrome virus potentially related to attenuation. Vet Microbiol. 2012, 157:50-60.
-
Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014, 10(7):e1004216.
-
Li J, Wang S, Li C, Wang C, Liu Y, Wang G, He X, Hu L, Liu Y, Cui M, Bi C, Shao Z, Wang X, Xiong T, Cai X, Huang L, Weng C. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet Microbiol.2017, 204:35-42.
-
Lindhaus W, Lindhaus B. Raetselhafte Schweinekrankheit. Prakt Tierarzt 1991, 5:423–5
-
Loula T. Mystery pig disease. Agri-practice. 1991, 12:23–34.
-
Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007, 120:217-29.
-
Lu W, Sun B, Mo J, Zeng X, Zhang G, Wang L, Zhou Q, Zhu L, Li Z, Xie Q, Bi Y, Ma J. Attenuation and immunogenicity of a live high pathogenic PRRSV vaccine candidate with a 32-amino acid deletion in the nsp2 protein. J Immunol Res. 2014, 2014:810523.
-
Lunney JK, Benfield DA, Rowland RR.Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154:1-6.
-
Mardassi H, Massie B, Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996, 221:98-112.
-
Martínez-Lobo FJ, Díez-Fuertes F, Segalés J, García-Artiga C, Simarro I, Castro JM, Prieto C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet Microbiol. 2011, 154:58-68.
-
Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008, 177:345-51.
-
Meng XJ, Paul PS, Halbur PG. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 1994, 75:1795-801.
-
Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 1995, 140:745-55.
-
Meredith MJ. Review of porcine reproductive and respiratory syndrome. Pig Disease Information Centre, University of Cambridge, Cambridge, England. 1-24.
-
Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993, 192:62-72.
-
Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus belongs to a new virus family, comprising lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus. Arch Virol Suppl. 1994, 9:441-8.
-
Meulenberg JJ, Petersen-den Besten A, De Kluyver EP, Moormann RJ, Schaaper WM, Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995, 206:155-63.
-
Morin M, Carpenter J, Poljack Z, Rivest J, Urizar L, Klopfenstein C, PRRS economic impact simulation tool for regional control and eradication projects in Canada. 2014 IPVS Congress. p 220.
-
Murtaugh MP1, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995, 140:1451-60.
-
Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154:18-30.
-
Nauwynck HJ, Van Gorp H, Vanhee M, Karniychuk U, Geldhof M, Cao A, Verbeeck M, Van Breedam W. Micro-dissecting the pathogenesis and immune response of PRRSV infection paves the way for more efficient PRRSV vaccines. Transbound Emerg Dis. 2012, 59 Suppl 1:50-4.
-
Nelson EA, Christopher-Hennings J, Drew T, Wensvoort G, Collins JE, Benfield DA. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993, 31:3184-9.
-
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005, 227:385-92.
-
Nieuwenhuis N, Duinhof TF, van Nes A. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet Rec. 2012, 170:225.
-
Ohlinger V.F., Pesch S., Bischoff C., History, occurrence, dynamics and current status of PRRS in Europe, Vet Res. 2000, 31: 86-87.
-
World Organisation for Animal Health; World Animal Health Information Database Interface; OIE 2013.
-
Pirtle EC, Beran GW. Stability of porcine reproductive and respiratory syndrome virus in the presence of fomites commonly found on farms. J Am Vet Med Assoc. 1996, 208:390-2. Plagemann PG. Porcine reproductive and respiratory syndrome virus: origin hypothesis. Emerg Infect Dis. 2003, 9:903-8.
-
Plagemann PG. Lactate dehydrogenase-elevating virus and related viruses. In: Fields Virology, 3rd ed. Ed. Fields BN, Knipe DM, Howley PM, et al. 1105-20.
-
Plana J, Vayreda M, Vilarrasa J, Bastons M, Rosell R, Martinez M, San Gabriel A, Pujols J, Badiola JL, Ramos JA, et al. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in Spain of the causative agent and experimental reproduction of the disease. Vet Microbiol. 1992, 33:203-11.
-
Pol JM, van Leengoed LA, Stockhofe N, Kok G, Wensvoort G. Dual infections of PRRSV/influenza or PRRSV/Actinobacillus pleuropneumoniae in the respiratory tract. Vet Microbiol. 1997, 55:259-64.
-
Reiner G. Genetic resistance – an alternative for controlling PRRS? Porcine Health Management 2016, 2:27.
-
Renukaradhya GJ, Alekseev K, Jung K, Fang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010, 23:457-66.
-
Salines M, Barnaud E, Andraud M, Eono F, Renson P, Bourry O, Pavio N, Rose N. Hepatitis E virus chronic infection of swine co-infected with Porcine Reproductive and Respiratory Syndrome Virus. Vet Res.2015, 6;46:55. Shibata I, Yazawa S, Ono M, Okuda Y. Experimental dual infection of specific pathogen-free pigs with porcine reproductive and respiratory syndrome virus and pseudorabies virus. J Vet Med B Infect Dis Vet Public Health. 2003, 50:14-9.
-
Shin JH, Kang YB, Kim YJ, Yeom SH, Kweon CH, Lee WY, Jean YH, Hwang EK, Rhee JC, An SH, Cho IS, Oh JS, Joo HS, Choi CS, Molitor TW. Sero-epidemiological studies on porcine reproductive and respiratory syndrome in Korea. I. Detection of indirect fluorescent antibodies. RDA J Agri Sci. 1993, 35: 572-6.
-
SIP Consultors. Repercusión económica del PRRS. Lleida, Spain. 2013.
-
Snijder EJ, Kikkert M, Fang Y. Arterivirus molecular biology and pathogenesis. J Gen Virol. 2013, 94:2141-63.
-
Solano GI, Segalés J, Collins JE, Molitor TW, Pijoan C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet Microbiol. 1997, 55:247-57.
-
Solano GI, Bautista E, Molitor TW, Segales J, Pijoan C. Effect of porcine reproductive and respiratory syndrome virus infection on the clearance of Haemophilus parasuis by porcine alveolar macrophages. Can J Vet Res. 1998, 62:251-6.
-
Stadejek T. Explore unexplored: origin of PRRSV revealed? In Proceedings of the European, Middle Eastern and Africa Society for Biopreservation and Biobanking (ESBB) conference. November 16-19. France.
-
Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999, 37:620-7.
-
Thacker EL, Thacker BJ, Young TF, Halbur PG. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000, 18:1244-52.
-
Thanawongnuwech R, Brown GB, Halbur PG, Roth JA, Royer RL, Thacker BJ. Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility to Streptococcus suis infection. Vet Pathol. 2000, 37: 143-52.
-
ThanawongnuwechR, Thacker B, Halbur P, Thacker EL. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin Diagn Lab Immunol. 2004, 11: 901-8.Thanawongnuwech R, Thacker EL, Halbur PG. Effect of Porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): In vitro comparisons with pulmonary alveolar macrophages (PAMs). Vet Immunol Immunopathol. 1997, 59:323-335.
-
Terpstra C, Wensvoort G, Pol JM. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch’s postulates fulfilled. Vet Q. 1991, 13:131-6.
-
Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One. 200, 2:e526.
-
Van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. 2003, 21:1952-7.
-
Van Gorp H, Van Breedam W, Delputte PL, Nauwynck HJ. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008, 89:2943-53.
-
Wang G, Song T, Yu Y, Liu Y, Shi W, Wang S, Rong F, Dong J, Liu H, Cai X, Zhou EM. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2011, 142:170-8.
-
Wensvoort G, Terpstra C, Pol JMA, Lask EA, Bloemraad M, de Kluyver EP, Kragten C, van Butten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PJM, Zetstra T, de Boer EA, Tibben AhJ, de Jong MF, van’r Veld P, Groenland GJR, van Gennep JA, Voets MTh, Verheijden JHM, Braamkamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet Q. 1991, 13:121–30.
-
Wesley RD, Mengeling WL, Lager KM. Prior infection of nursery-age pigs with porcine reproductive and respiratory syndrome virus does not affect the outcome of transmissible gastroenteritis virus challenge. J Vet Diagn Invest. 1998, 10:221-8.
-
White M. Blue ear disease of pigs. Vet Rec. 1991, 128:574.
-
White M. The clinical signs and symptoms of “blue eared pig disease”. Pig Vet J. 1991, 28:62-8.
-
Wills RW, Gray JT, Fedorka-Cray PJ, Yoon KJ, Ladely S, Zimmerman JJ. Synergism between porcine reproductive and respiratory syndrome virus (PRRSV) and Salmonella choleraesuis in swine. Vet Microbiol. 2000, 71:177-92.
-
pig333.com/prrs/prrsv-infection-in-france-clinical-and-economic-impact_9563/
-
thepigsite.com/articles/4490/danish-pig-research-centreannual-report-2012-prrs
-
Yamane I, Kure K, Ishikawa H, Takagi M, Miyazaki A, Suzuki T, Shibahara T, Kubo M, Kobayashi H, Kokuho T, Tsunemitsu H. Estimation of economic loss due to porcine reproductive and respiratory syndrome in Japan.
-
Yu J, Wu J, Zhang Y, Guo L, Cong X, Du Y, Li J, Sun W, Shi J, Peng J, Yin F, Wang D, Zhao P, Wang J. Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs. Vet Microbiol.2012, 158:316-21.
-
Yun SI, Lee YM. Overview: Replication of porcine reproductive and respiratory syndrome virus. J Microbiol. 2013, 51:711-23.
-
Zhang Q, Yoo D. PRRS virus receptors and their role for pathogenesis. Vet Microbiol. 2015, 177:229-41.
-
Zimmerman J, Yoon K-J, Wills RW, Swenson SL. General overview of PRRSV: A perspective from the United States. Vet Microbiol. 1997, 55:187-96.
-
Zhou Z, Ni J, Cao Z, Han X, Xia Y, Zi Z, Ning K, Liu Q, Cai L, Qiu P, Deng X, Hu D, Zhang Q, Fan Y, Wu J, Wang L, Zhang M, Yu X, Zhai X, Tian K. The epidemic status and genetic diversity of 14 highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) isolates from China in 2009. Vet Microbiol. 2011, 150:257-69.
-
Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: 10th ed. Diseases of swine, Ed. Wiley-Blackwell. 2012, 31:463-86.
-
Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol. 2000, 145:2421-9.
-
Baron T, Albina E, Leforban Y, Madec F, Guilmoto H, Plana Duran J, Vannier P. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann Rech Vet. 1992, 23:161-6.
-
Benfield DA, Nelson E, Collins JE, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 1992. 4, 127-133.
-
Benfield D, Nelson J, Rossow K, Nelson C, Steffen M, Rowland R. Diagnosis of persistent or prolonged porcine reproductive and respiratory syndrome virus infections Vet Res. 2000, 31:71.
-
Blaha T. The ”colorful” epidemiology of PRRS. Vet Res BioMed Central. 2000, 31:77-83.
-
Brockmeier SL, Palmer MV, Bolin SR. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. Am J Vet Res. 2000, 61:892-9.
-
Carman S, Sanford SE, Dea S. Assessment of seropositivity to porcine reproductive and respiratory syndrome (PRRS) virus in swine herds in Ontario–1978 to 1982. Can Vet J. 1995, 36:776-7.
-
Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992, 4:117-26.
-
Cortey M, Díaz I, Martín-Valls GE, Mateu E. Next-generation sequencing as a tool for the study of Porcine reproductive and respiratory syndrome virus (PRRSV) macro- and micro- molecular epidemiology. Vet Microbiol. 2017. doi: 10.1016/j.vetmic.2017.02.002.
-
Delrue I, Van Gorp H, Van Doorsselaere J, Delputte PL, Nauwynck HJ. Susceptible cell lines for the production of porcine reproductive and respiratory syndrome virus by stable transfection of sialoadhesin and CD163. BMC Biotechnol. 2010, 29:10:48.
-
Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006, 351:249-59.
-
Dobrescu I, Levast B, Lai K, Delgado-Ortega M, Walker S, Banman S, Townsend H, Simon G, Zhou Y, Gerdts V, Meurens F. In vitro and ex vivo analyses of co-infections with swine influenza and porcine reproductive and respiratory syndrome viruses. Vet Microbiol.2014, 169:18-32.
-
Duan X, Nauwynck HJ, Pensaert MB. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet Microbiol. 1997, 56:9-19.
-
Fan P, Wei Y, Guo L, Wu H, Huang L, Liu J, Liu C. Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Virol J.2013, 10:265.
-
Forsberg R, Oleksiewicz MB, Petersen AM, Hein J, Bøtner A, Storgaard T. A molecular clock dates the common ancestor of European-type porcine reproductive and respiratory syndrome virus at more than 10 years before the emergence of disease. Virology. 2001, 289:174-9.
-
Gómez-Laguna J, Salguero FJ, Pallarés FJ, Carrasco L. Immunopathogenesis of porcine reproductive and respiratory syndrome in the respiratory tract of pigs. Vet J. 2013, 195:148-55.
-
Harms PA, Sorden SD, Halbur PG, Bolin SR, Lager KM, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet Pathol. 2001, 38:528-39.
-
He Q, Li Y, Zhou L, Ge X, Guo X, Yang H. Both Nsp1β and Nsp11 are responsible for differential TNF-α production induced by porcine reproductive and respiratory syndrome virus strains with different pathogenicity in vitro. Virus Res. 2015, 201:32-40.
-
Hill H. Overview and history of Mystery Swine Disease (swine infertility/respiratory syndrome). Proceedings of the Mystery Swine Disease Committee Meeting, Livestock Conversation Institute, Denver, CO. 1990, 29–31.
-
Hirose 0, Kudo H, Yoshizawa S, Hiroike T, Nakane T. Prevalence of porcine reproductive and respiratory syndrome virus in Chiba prefecture. J Jpn Vet Med Assoc. 1995, 48:650-3.
-
Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL and Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013, 21:72-84.
-
Hirose O, Shibata I, Kudou H, Samegai Y, Yoshizawa S, Ono M, Nishimura M, Hiroike T, Kageyama K, Sakano T. Experimental infection of SPF piglets with porcine reproductive and respiratory syndrome (PRRS) viruses isolated from two farms. J Vet Med Sci. 1995, 57:991-5.
-
Jung K, Renukaradhya GJ, Alekseev KP, Fang Y, Tang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009, 90:2713-23.
-
Karniychuk UU, Nauwynck HJ. Quantitative changes of sialoadhesin and CD163 positive macrophages in the implantation sites and organs of porcine embryos/fetuses during gestation. Placenta. 2009, 30:497-500.
-
Karniychuk UU, Geldhof M, Vanhee M, Van Doorsselaere J, Saveleva TA, Nauwynck HJ. Pathogenesis and antigenic characterization of a new East European subtype 3 porcine reproductive and respiratory syndrome virus isolate. BMC Vet Res. 2010, 4:6:30.
-
Karniychuk UU, Nauwynck HJ. Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet Res. 2013, 44:95.
-
Kavanová L, Matiašková K, Levá L, Štěpánová H, Nedbalcová K, Matiašovic J, Faldyna M, Salát J. Concurrent infection with porcine reproductive and respiratory syndrome virus and Haemophilus parasuis in two types of porcine macrophages: apoptosis, production of ROS and formation of multinucleated giant cells. Vet Res.2017, 48:28.
-
Kitikoon P, Vincent AL, Jones KR, Nilubol D, Yu S, Janke BH, Thacker BJ, Thacker EL. Vaccine efficacy and immune response to swine influenza virus challenge in pigs infected with porcine reproductive and respiratory syndrome virus at the time of SIV vaccination. Vet Microbiol. 2009, 139:235-44.
-
Keffaber KK. Reproductive failure of unknown etiology. AASP. 1989, 1: 2–10.
-
Lawson SR, Rossow KD, Collins JE, Benfield DA, Rowland RR. Porcine reproductive and respiratory syndrome virus infection of gnotobiotic pigs: sites of virus replication and co-localization with MAC-387 staining at 21 days post-infection. Virus Res. 1997, 51:105-13.
-
Leng X, Li Z, Xia M, Li X, Wang F, Wang W, Zhang X, Wu H. Mutations in the genome of the highly pathogenic porcine reproductive and respiratory syndrome virus potentially related to attenuation. Vet Microbiol. 2012, 157:50-60.
-
Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014, 10(7):e1004216.
-
Li J, Wang S, Li C, Wang C, Liu Y, Wang G, He X, Hu L, Liu Y, Cui M, Bi C, Shao Z, Wang X, Xiong T, Cai X, Huang L, Weng C. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet Microbiol.2017, 204:35-42.
-
Lindhaus W, Lindhaus B. Raetselhafte Schweinekrankheit. Prakt Tierarzt 1991, 5:423–5
-
Loula T. Mystery pig disease. Agri-practice. 1991, 12:23–34.
-
Loving CL, Brockmeier SL, Sacco RE. Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology. 2007, 120:217-29.
-
Lu W, Sun B, Mo J, Zeng X, Zhang G, Wang L, Zhou Q, Zhu L, Li Z, Xie Q, Bi Y, Ma J. Attenuation and immunogenicity of a live high pathogenic PRRSV vaccine candidate with a 32-amino acid deletion in the nsp2 protein. J Immunol Res. 2014, 2014:810523.
-
Lunney JK, Benfield DA, Rowland RR.Porcine reproductive and respiratory syndrome virus: an update on an emerging and re-emerging viral disease of swine. Virus Res. 2010, 154:1-6.
-
Mardassi H, Massie B, Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996, 221:98-112.
-
Martínez-Lobo FJ, Díez-Fuertes F, Segalés J, García-Artiga C, Simarro I, Castro JM, Prieto C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet Microbiol. 2011, 154:58-68.
-
Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008, 177:345-51.
-
Meng XJ, Paul PS, Halbur PG. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 1994, 75:1795-801.
-
Meng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 1995, 140:745-55.
-
Meredith MJ. Review of porcine reproductive and respiratory syndrome. Pig Disease Information Centre, University of Cambridge, Cambridge, England. 1-24.
-
Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993, 192:62-72.
-
Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus belongs to a new virus family, comprising lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus. Arch Virol Suppl. 1994, 9:441-8.
-
Meulenberg JJ, Petersen-den Besten A, De Kluyver EP, Moormann RJ, Schaaper WM, Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995, 206:155-63.
-
Morin M, Carpenter J, Poljack Z, Rivest J, Urizar L, Klopfenstein C, PRRS economic impact simulation tool for regional control and eradication projects in Canada. 2014 IPVS Congress. p 220.
-
Murtaugh MP1, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995, 140:1451-60.
-
Murtaugh MP, Stadejek T, Abrahante JE, Lam TT, Leung FC. The ever-expanding diversity of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154:18-30.
-
Nauwynck HJ, Van Gorp H, Vanhee M, Karniychuk U, Geldhof M, Cao A, Verbeeck M, Van Breedam W. Micro-dissecting the pathogenesis and immune response of PRRSV infection paves the way for more efficient PRRSV vaccines. Transbound Emerg Dis. 2012, 59 Suppl 1:50-4.
-
Nelson EA, Christopher-Hennings J, Drew T, Wensvoort G, Collins JE, Benfield DA. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993, 31:3184-9.
-
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005, 227:385-92.
-
Nieuwenhuis N, Duinhof TF, van Nes A. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet Rec. 2012, 170:225.
-
Ohlinger V.F., Pesch S., Bischoff C., History, occurrence, dynamics and current status of PRRS in Europe, Vet Res. 2000, 31: 86-87.
-
World Organisation for Animal Health; World Animal Health Information Database Interface; OIE 2013.
-
Pirtle EC, Beran GW. Stability of porcine reproductive and respiratory syndrome virus in the presence of fomites commonly found on farms. J Am Vet Med Assoc. 1996, 208:390-2. Plagemann PG. Porcine reproductive and respiratory syndrome virus: origin hypothesis. Emerg Infect Dis. 2003, 9:903-8.
-
Plagemann PG. Lactate dehydrogenase-elevating virus and related viruses. In: Fields Virology, 3rd ed. Ed. Fields BN, Knipe DM, Howley PM, et al. 1105-20.
-
Plana J, Vayreda M, Vilarrasa J, Bastons M, Rosell R, Martinez M, San Gabriel A, Pujols J, Badiola JL, Ramos JA, et al. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in Spain of the causative agent and experimental reproduction of the disease. Vet Microbiol. 1992, 33:203-11.
-
Pol JM, van Leengoed LA, Stockhofe N, Kok G, Wensvoort G. Dual infections of PRRSV/influenza or PRRSV/Actinobacillus pleuropneumoniae in the respiratory tract. Vet Microbiol. 1997, 55:259-64.
-
Reiner G. Genetic resistance – an alternative for controlling PRRS? Porcine Health Management 2016, 2:27.
-
Renukaradhya GJ, Alekseev K, Jung K, Fang Y, Saif LJ. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010, 23:457-66.
-
Salines M, Barnaud E, Andraud M, Eono F, Renson P, Bourry O, Pavio N, Rose N. Hepatitis E virus chronic infection of swine co-infected with Porcine Reproductive and Respiratory Syndrome Virus. Vet Res.2015, 6;46:55. Shibata I, Yazawa S, Ono M, Okuda Y. Experimental dual infection of specific pathogen-free pigs with porcine reproductive and respiratory syndrome virus and pseudorabies virus. J Vet Med B Infect Dis Vet Public Health. 2003, 50:14-9.
-
Shin JH, Kang YB, Kim YJ, Yeom SH, Kweon CH, Lee WY, Jean YH, Hwang EK, Rhee JC, An SH, Cho IS, Oh JS, Joo HS, Choi CS, Molitor TW. Sero-epidemiological studies on porcine reproductive and respiratory syndrome in Korea. I. Detection of indirect fluorescent antibodies. RDA J Agri Sci. 1993, 35: 572-6.
-
SIP Consultors. Repercusión económica del PRRS. Lleida, Spain. 2013.
-
Snijder EJ, Kikkert M, Fang Y. Arterivirus molecular biology and pathogenesis. J Gen Virol. 2013, 94:2141-63.
-
Solano GI, Segalés J, Collins JE, Molitor TW, Pijoan C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet Microbiol. 1997, 55:247-57.
-
Solano GI, Bautista E, Molitor TW, Segales J, Pijoan C. Effect of porcine reproductive and respiratory syndrome virus infection on the clearance of Haemophilus parasuis by porcine alveolar macrophages. Can J Vet Res. 1998, 62:251-6.
-
Stadejek T. Explore unexplored: origin of PRRSV revealed? In Proceedings of the European, Middle Eastern and Africa Society for Biopreservation and Biobanking (ESBB) conference. November 16-19. France.
-
Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999, 37:620-7.
-
Thacker EL, Thacker BJ, Young TF, Halbur PG. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000, 18:1244-52.
-
Thanawongnuwech R, Brown GB, Halbur PG, Roth JA, Royer RL, Thacker BJ. Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility to Streptococcus suis infection. Vet Pathol. 2000, 37: 143-52.
-
ThanawongnuwechR, Thacker B, Halbur P, Thacker EL. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin Diagn Lab Immunol. 2004, 11: 901-8.Thanawongnuwech R, Thacker EL, Halbur PG. Effect of Porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): In vitro comparisons with pulmonary alveolar macrophages (PAMs). Vet Immunol Immunopathol. 1997, 59:323-335.
-
Terpstra C, Wensvoort G, Pol JM. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch’s postulates fulfilled. Vet Q. 1991, 13:131-6.
-
Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X, Liu D, Zhang S, Deng X, Ding Y, Yang L, Zhang Y, Xiao H, Qiao M, Wang B, Hou L, Wang X, Yang X, Kang L, Sun M, Jin P, Wang S, Kitamura Y, Yan J, Gao GF. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One. 200, 2:e526.
-
Van der Linden IF, Voermans JJ, van der Linde-Bril EM, Bianchi AT, Steverink PJ. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. 2003, 21:1952-7.
-
Van Gorp H, Van Breedam W, Delputte PL, Nauwynck HJ. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008, 89:2943-53.
-
Wang G, Song T, Yu Y, Liu Y, Shi W, Wang S, Rong F, Dong J, Liu H, Cai X, Zhou EM. Immune responses in piglets infected with highly pathogenic porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2011, 142:170-8.
-
Wensvoort G, Terpstra C, Pol JMA, Lask EA, Bloemraad M, de Kluyver EP, Kragten C, van Butten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PJM, Zetstra T, de Boer EA, Tibben AhJ, de Jong MF, van’r Veld P, Groenland GJR, van Gennep JA, Voets MTh, Verheijden JHM, Braamkamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet Q. 1991, 13:121–30.
-
Wesley RD, Mengeling WL, Lager KM. Prior infection of nursery-age pigs with porcine reproductive and respiratory syndrome virus does not affect the outcome of transmissible gastroenteritis virus challenge. J Vet Diagn Invest. 1998, 10:221-8.
-
White M. Blue ear disease of pigs. Vet Rec. 1991, 128:574.
-
White M. The clinical signs and symptoms of “blue eared pig disease”. Pig Vet J. 1991, 28:62-8.
-
Wills RW, Gray JT, Fedorka-Cray PJ, Yoon KJ, Ladely S, Zimmerman JJ. Synergism between porcine reproductive and respiratory syndrome virus (PRRSV) and Salmonella choleraesuis in swine. Vet Microbiol. 2000, 71:177-92.
-
pig333.com/prrs/prrsv-infection-in-france-clinical-and-economic-impact_9563/
-
thepigsite.com/articles/4490/danish-pig-research-centreannual-report-2012-prrs
-
Yamane I, Kure K, Ishikawa H, Takagi M, Miyazaki A, Suzuki T, Shibahara T, Kubo M, Kobayashi H, Kokuho T, Tsunemitsu H. Estimation of economic loss due to porcine reproductive and respiratory syndrome in Japan.
-
Yu J, Wu J, Zhang Y, Guo L, Cong X, Du Y, Li J, Sun W, Shi J, Peng J, Yin F, Wang D, Zhao P, Wang J. Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs. Vet Microbiol.2012, 158:316-21.
-
Yun SI, Lee YM. Overview: Replication of porcine reproductive and respiratory syndrome virus. J Microbiol. 2013, 51:711-23.
-
Zhang Q, Yoo D. PRRS virus receptors and their role for pathogenesis. Vet Microbiol. 2015, 177:229-41.
-
Zimmerman J, Yoon K-J, Wills RW, Swenson SL. General overview of PRRSV: A perspective from the United States. Vet Microbiol. 1997, 55:187-96.
-
Zhou Z, Ni J, Cao Z, Han X, Xia Y, Zi Z, Ning K, Liu Q, Cai L, Qiu P, Deng X, Hu D, Zhang Q, Fan Y, Wu J, Wang L, Zhang M, Yu X, Zhai X, Tian K. The epidemic status and genetic diversity of 14 highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) isolates from China in 2009. Vet Microbiol. 2011, 150:257-69.
-
Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus). In: 10th ed. Diseases of swine, Ed. Wiley-Blackwell. 2012, 31:463-86.